
The biosynthesis of serine (amino acid), purines, heme (part of hemoglobin found in the blood), and also glutathione (coenzyme) all require glycine. Glycine (Gly) is unreactive when it is in proteins. Lysine structure Amino Acids with Special CasesĬysteine (Cys) is able to form disulfide bridges between to peptide chains and also form loops within a singular chain. Specifically, it binds to histone acetyl transferases which alter the transcription of certain genes. It plays an important role in the way that histones function. Lysine (Lys) is in the binding enzymes to coenzymes. Due to this property, histidine can combine into enzymes involved in the metabolism of proteins, carbohydrates, and nucleic acids. Histidine (His) within proteins acts as both a proton acceptor and donor. Glutamic acid structure Amino Acid Basic Side ChainsĪrginine (Arg) is produced when proteins are digested within our bodies, and it is converted into nitric oxide (responsible for relaxing blood vessels).
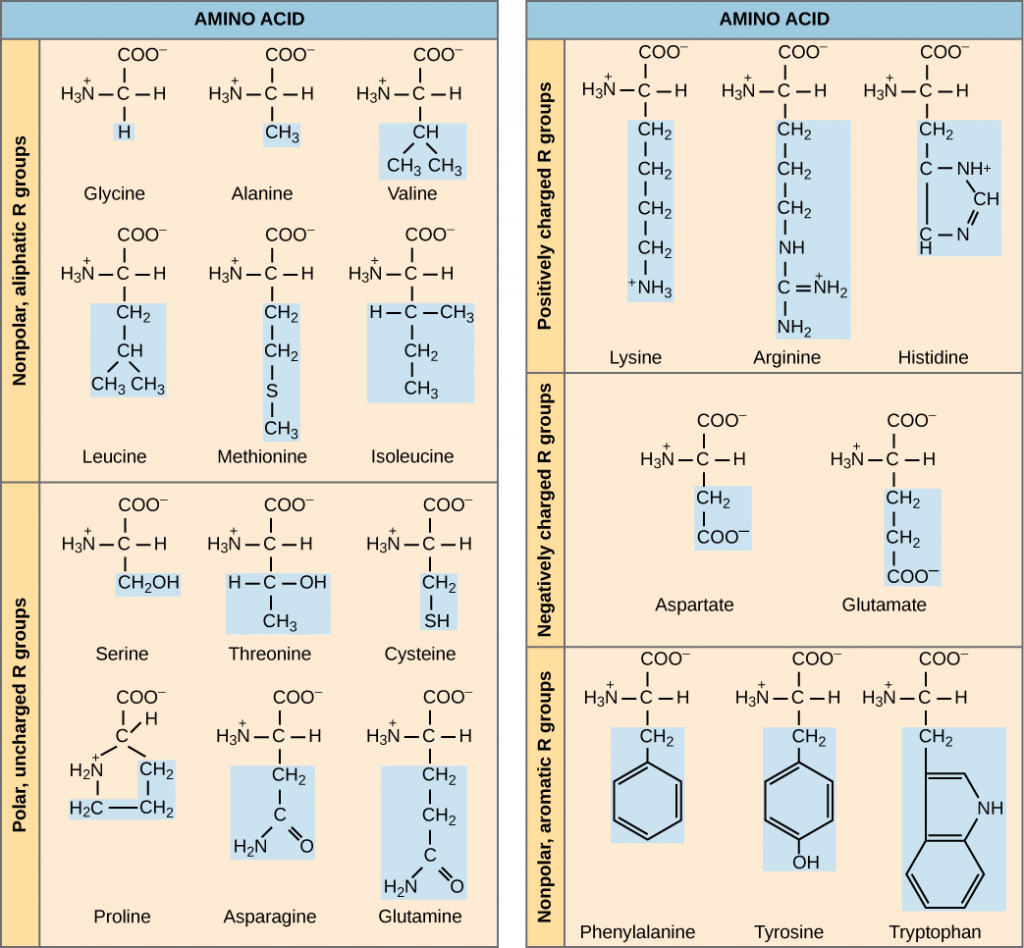
Glutamic acid (Glu) is similar to aspartic acid and is highly soluble in water. Threonine structure Amino Acid Acidic Side ChainsĪspartic acid (Asp) is water soluble, which then allows it to be near the active sites of enzymes. However, the exact effect has not yet been determined. Threonine (Thr) is within reactions in bacteria and metabolic rate in animals. Serine (Ser) is involved in biosynthesis of metabolites and is important in the catalytic function of enzymes. The reason glutamine can remove toxic ammonia is because its carboxyl side chain can act as a donor and acceptor for ammonia (this then allows for the safe transport of ammonia in our bodies). It is responsible for regulating toxic ammonia and urea in our bodies. Glutamine (Gln) is the most abundant amino acid in our bodies, and it performs several functions. This means that their side chains are neither acidic nor basic.Īsparagine (Asn) is responsible for removing ammonia (a toxic chemical) from our bodies. The amino acids below all have side chains that are polar neutral. Tyrosine structure Amino Acid Neutral Side Chains
STRUCTURE OF AN AMINO ACID SKIN
It is within the adrenal hormones (epinephrine and norepinephrine), the thyroid hormones (thyroxine), and melanin (responsible for hair and skin pigment).

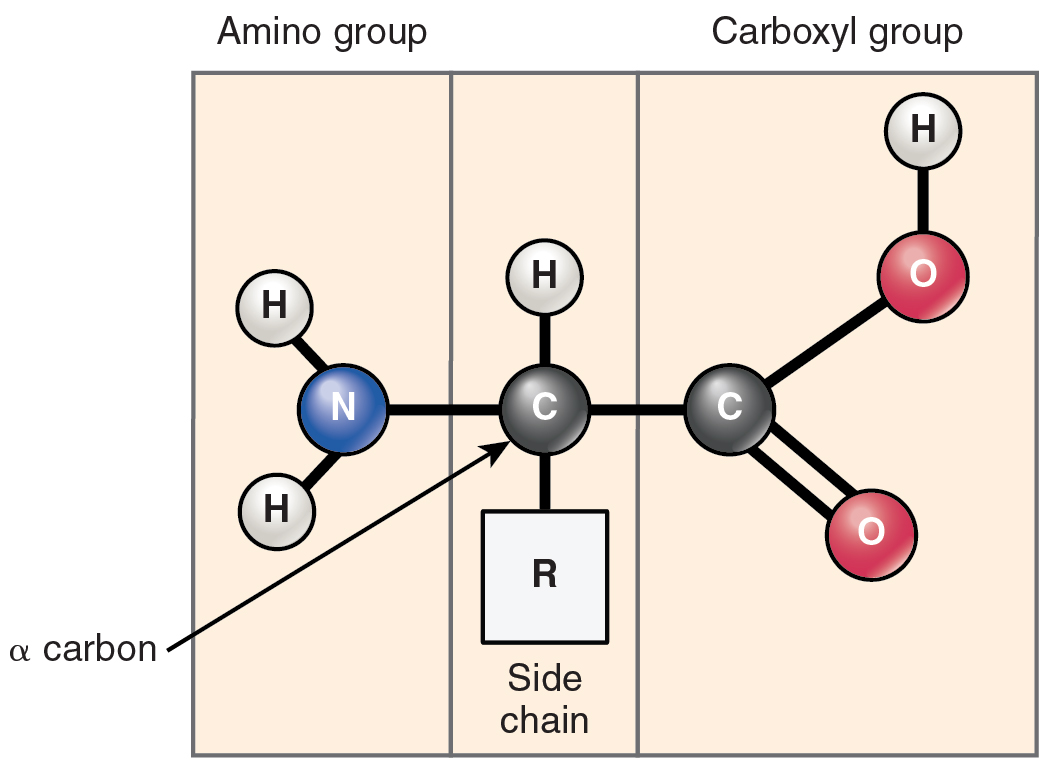
Tyrosine (Tyr) is also an important precursor to vital hormones within our bodies. Tryptophan (Trp) can break down in the human gut. Phenylalanine (Phe) can break down into tyrosine within the body. Methionine (Met) prevents the accumulation of fat in the liver and detoxifies wastes and toxins. However, it is degraded through the use of enzymes. Leucine (Leu) is similar to valine where it is degraded into simpler compounds within the body. The means the main difference between the various amino acids lies in the structure of the ' R ' group. Isoleucine (Ile) is important when the tertiary structure of a protein it is included in is being determined. The 20 naturally occurring -amino acids used by cells to synthesise proteins can be generally represented by the generic formula shown above. Valine (Val) is able to break down into simpler compounds within our bodies. This property allows for alanine to create an elongated structure that is equally flexible and stretch resistant. This property makes them “water fearing” or unable to dissolve in water.Īlanine (Ala) is the first hydrophobic amino acid, and it has low reactivity. The amino acids below all have a side chain that then makes them to be hydrophobic. Amino Acid Side Chains Amino Acid Hydrophobic Side Chains However, selenocysteine is not part of the 20 essential amino acids that are in proteins. This also affects the 3D structure and properties that a protein has. As a result, these three properties affect the way they interact with their surroundings within polypeptides and proteins. Their different side chains are categorized by charge, hydrophobicity (how they react with water), and also polarity. In particular, glycine has hydrogen as its R group. The simplest or smallest amino acid is glycine. To repeat, the only difference in their structures is their R groups.

Amino acid structureĢ0 amino acids make up all proteins. However, amino acids are different from each other based on the composition of their R groups. In each amino acid, an amino group and a carboxylic acid group attach to a carbon. They are important pieces of our bodies and assist in many processes such as protein synthesis. Topics Covered in Other ArticlesĪmino acids are the building blocks for polypeptides and proteins. \)Īmino acids are the monomers that make up proteins.In this tutorial, you will learn about the 20 amino acid structures, along with their important biochemical properties.


 0 kommentar(er)
0 kommentar(er)
